Ivdr Gspr Checklist Template
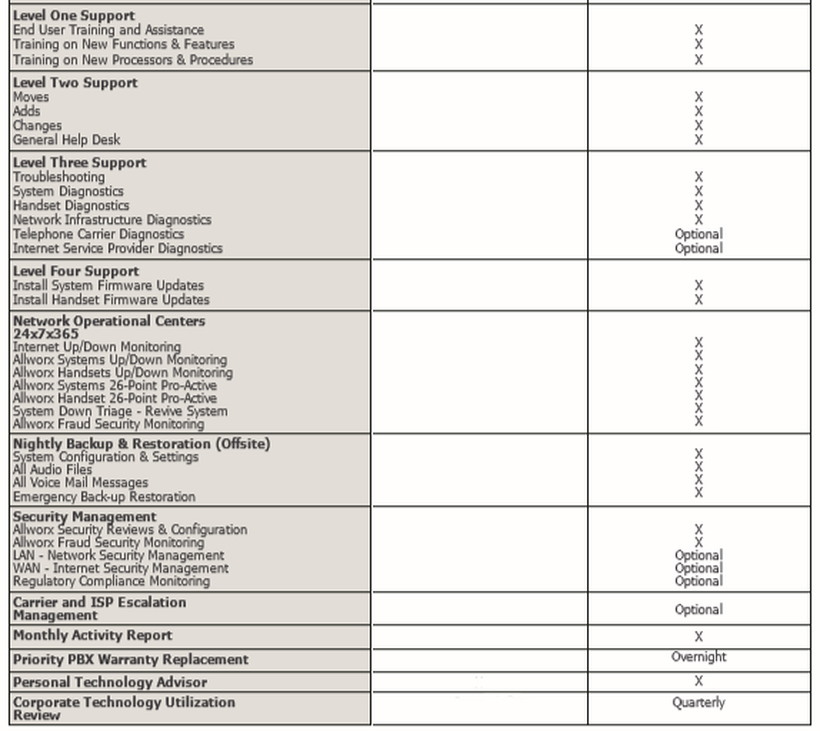
Ivdr Gspr Checklist Template - Web the new eu mdr and eu ivdr, which repealed the medical devices directive 93/42/eec, active implantable medical devices directive 90/385/eec, and in vitro diagnostic medical devices directive 98/79/ec, were released on april 5, 2017. Here’s a quick checklist to get you started on the path to compliance: Web are shifting focus to the sister in vitro diagnostic regulation (ivdr) which has rolling effective dates starting in may 2022. Web a checklist that manufacturers may complete to demonstrate how they have complied with the gsprs for an ivd, and where the associated evidence can be found, is available from nsf (see end of paper). Web bundle tech file (template and checklist) + gspr + doc. General safety and performance (gspr) checklist. The expanded 2nd edition of this ebook includes a detailed summary of the ivdr gspr regulations in addition to those of the mdr. Before placing in vitro diagnostic (ivd) devices on the market, most manufacturers will need their technical documentation assessed by a notified body. Helpful suggestions are included on the available methods that could be used to demonstrate conformity to each gspr. For any issue, please contact us. Mdr “general safety and performance requirements” annex i. Web easily identify the standards or other solutions that are relevant to your device, and to each gspr. If notified bodies or other technical specialists in this sector identify any errors in their work, no objections will be heard. Ce 2797 throughout this guide, our notified body is referenced using its assigned. Designed to be easy to use and follow, the template will save you many hours, headaches and potential mistakes. Before placing in vitro diagnostic (ivd) devices on the market, most manufacturers will need their technical documentation assessed by a notified body. Web the new eu mdr and eu ivdr, which repealed the medical devices directive 93/42/eec, active implantable medical devices. General safety and performance (gspr) checklist. No refund possible after purchase. Web are shifting focus to the sister in vitro diagnostic regulation (ivdr) which has rolling effective dates starting in may 2022. Like the mdr, the ivdr also includes new general safety and performance requirements (gspr). Web bundle tech file (template and checklist) + gspr + doc. It is specifically designed for the area of mdd “essential requirements” vs. This checklist once filled out establishes the objective. Web bundle tech file (template and checklist) + gspr + doc. Although the regulation (eu) 2017/746 in in vitro diagnostic medical devices (ivdr) presents several challenges for manufacturers, the. Web the new eu mdr and eu ivdr, which repealed the. Web are shifting focus to the sister in vitro diagnostic regulation (ivdr) which has rolling effective dates starting in may 2022. Mdr “general safety and performance requirements” annex i. Web mdrg is currently creating an ivdr general safety & performance requirements checklist that contains a full table of the requirements, along with a list of applicable standards. The general safety. Mdr “general safety and performance requirements” annex i. Get your performance evaluation reports (per) right. The general safety and performance requirements (gspr) checklist is a checklist against annex i of the in vitro diagnostic device regulations (ivdr) eu 2017/746. Checklist ivdr technical documentation (short) download. No refund possible after purchase. Web the new eu mdr and eu ivdr, which repealed the medical devices directive 93/42/eec, active implantable medical devices directive 90/385/eec, and in vitro diagnostic medical devices directive 98/79/ec, were released on april 5, 2017. Web the gspr is known as general safety and performance requirements are listed in annex i of eu mdr 2017/745 and eu ivdr 2017/746. Helpful. If notified bodies or other technical specialists in this sector identify any errors in their work, no objections will be heard. Before placing in vitro diagnostic (ivd) devices on the market, most manufacturers will need their technical documentation assessed by a notified body. With this bundle download templates for: Checklist ivdr technical documentation (short) download. No refund possible after purchase. Web a checklist that manufacturers may complete to demonstrate how they have complied with the gsprs for an ivd, and where the associated evidence can be found, is available from nsf (see end of paper). Checklist ivdr technical documentation (extensive) download. With this bundle download templates for: Ce 2797 throughout this guide, our notified body is referenced using its assigned. For any issue, please contact us. Web mdrg is currently creating an ivdr general safety & performance requirements checklist that contains a full table of the requirements, along with a list of applicable standards. Common specifications the european commission provides common specifications to the ivdr as a means of complying with the legal obligations applicable to a device, Web are. Before placing in vitro diagnostic (ivd) devices on the market, most manufacturers will need their technical documentation assessed by a notified body. Although the regulation (eu) 2017/746 in in vitro diagnostic medical devices (ivdr) presents several challenges for manufacturers, the. Here’s a quick checklist to get you started on the path to compliance: Helpful suggestions are included on the available methods that could be used to demonstrate conformity to each gspr. The expanded 2nd edition of this ebook includes a detailed summary of the ivdr gspr regulations in addition to those of the mdr. Web standards, training, testing, assessment and certification | bsi It is specifically designed for the area of mdd “essential requirements” vs. With this bundle download templates for: Web i'm not sure why you're not able to take your existing essential requirements checklist and paste in the requirements from the annex of the regulations to create a gspr checklist? This checklist once filled out establishes the objective. Mdr “general safety and performance requirements” annex i. Web requirements (gspr) within the ivdr. The general safety and performance requirements (gspr) checklist is a checklist against annex i of the in vitro diagnostic device regulations (ivdr) eu 2017/746. If notified bodies or other technical specialists in this sector identify any errors in their work, no objections will be heard. No refund possible after purchase. Web the gspr is known as general safety and performance requirements are listed in annex i of eu mdr 2017/745 and eu ivdr 2017/746. Checklist ivdr technical documentation (short) download. Declaration of conformity (doc) all these are compatible with mdr 2017/745. For any issue, please contact us. Web easily identify the standards or other solutions that are relevant to your device, and to each gspr. Declaration of conformity (doc) all these are compatible with mdr 2017/745. Before placing in vitro diagnostic (ivd) devices on the market, most manufacturers will need their technical documentation assessed by a notified body. Web buy ivdr gspr checklist template * the ivdr gspr checklist template was prepared exclusively on the basis of our expertise and skills by our technical specialists. The general safety and performance requirements (gspr) checklist is a checklist against annex i of the in vitro diagnostic device regulations (ivdr) eu 2017/746. Web easily identify the standards or other solutions that are relevant to your device, and to each gspr. It is specifically designed for the area of mdd “essential requirements” vs. This checklist once filled out establishes the objective. Although the regulation (eu) 2017/746 in in vitro diagnostic medical devices (ivdr) presents several challenges for manufacturers, the. Mdr “general safety and performance requirements” annex i. Web qualitymeddev has made available a gspr checklist that will help you to ensure compliance of your devices and related documentation with the safety and performance requirements for the eu mdr 2017/745. No refund possible after purchase. Here’s a quick checklist to get you started on the path to compliance: One aspect of ivdr that has the potential to cause delays in timelines and product readiness is the performance evaluation report (per). Like the mdr, the ivdr also includes new general safety and performance requirements (gspr). Common specifications the european commission provides common specifications to the ivdr as a means of complying with the legal obligations applicable to a device, The gspr has 23 requirements under mdr and 20 requirements under ivdr.Ivdr Gspr Checklist Template Portal Tutorials
UDI Checklist MDR 2017/745 Easy Medical Device School
Ivdr Gspr Checklist Template Portal Tutorials
Ivdr Gspr Checklist Template Portal Tutorials
Pin on Help
Ivdr Gspr Checklist Template Portal Tutorials
Comparative table GSPR Essential Requirements (v3) Formations sur
GSPR General Safety And Performance Requirements [EU MDR & IVDR]
Ivdr Gspr Checklist Template Portal Tutorials
GSPR General Safety And Performance Requirements [EU MDR & IVDR]
Web A Checklist That Manufacturers May Complete To Demonstrate How They Have Complied With The Gsprs For An Ivd, And Where The Associated Evidence Can Be Found, Is Available From Nsf (See End Of Paper).
The Expanded 2Nd Edition Of This Ebook Includes A Detailed Summary Of The Ivdr Gspr Regulations In Addition To Those Of The Mdr.
For Any Issue, Please Contact Us.
Tips, Checklists, And Templates From Seasoned Medical Device Professionals Available At Your Fingertips.
Related Post:







![GSPR General Safety And Performance Requirements [EU MDR & IVDR]](https://easymedicaldevice.com/wp-content/uploads/2021/02/GSPR-Article-Web-Medium_LOW-1024x640.jpg)

![GSPR General Safety And Performance Requirements [EU MDR & IVDR]](https://easymedicaldevice.com/wp-content/uploads/2019/11/GSPR-capture.png)